
Deep imaging
CASS microscopy
Optical microscopy suffers from a loss of resolving power when imaging targets are embedded in thick scattering media because of the dominance of strong multiple-scattered waves over waves scattered only a single time by the targets. We developed an approach that maintains full optical resolution when imaging deep within scattering media. We use both time-gated detection and spatial input–output correlation to identify those reflected waves that conserve in-plane momentum, which is a property of single-scattered waves. By implementing a superradiance-like collective accumulation of the single scattering (CASS) waves, we enhance the ratio of the single scattering signal to the multiple scattering background by more than three orders of magnitude. An imaging depth of 11.5 times the scattering mean free path is achieved with a near-diffraction-limited resolution of 1.5 μm. Our method of distinguishing single- from multiple-scattered waves will open new routes to deep-tissue imaging and studying the physics of the interaction of light with complex media.

Experimental schematic diagram of the CASS microscope. SLD, diode laser; OL, objective lens; BS1, BS2 and BS3, beamsplitters; SLM, spatial light modulator (working in reflection mode, but indicated here as a transmission mode for simplicity); DG, diffraction grating (an aperture was used to select the first-order diffracted wave); SM, path length scanning mirror; CCD, charge-coupled device camera. For clarity, red, green and dark gold are used to indicate incident, reflected and reference waves, respectively, although their wavelengths are the same.
Reference:
Sungsam Kang, Seungwon Jeong, et al., "Imaging deep within a scattering medium using collective accumulation of single-scattered waves," Nature Photonics 9, 253-258 (9 Mar 2015)

CLASS microscopy
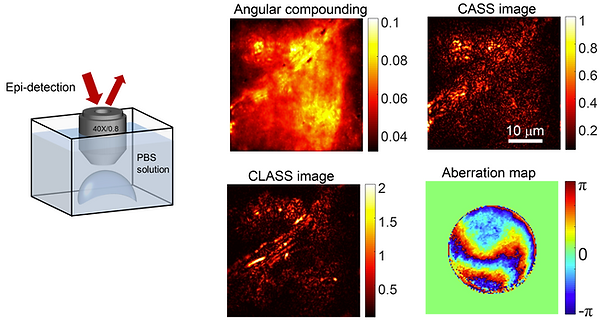
Thick biological tissues give rise to not only the multiple scattering of incoming light waves, but also the aberrations of remaining signal waves. The challenge for existing optical microscopy methods to overcome both problems simultaneously has limited sub-micron spatial resolution imaging to shallow depths. Here we present an optical coherence imaging method that can identify aberrations of waves incident to and reflected from the samples separately, and eliminate such aberrations even in the presence of multiple light scattering. The proposed method records the time-gated complex-feld maps of backscattered waves over various illumination channels, and performs a closed-loop optimization of signal waves for both forward and phase-conjugation processes. We demonstrated the enhancement of the Strehl ratio by more than 500 times, an order of magnitude or more improvement over conventional adaptive optics, and achieved a spatial resolution of 600 nm up to an imaging depth of seven scattering mean free paths.
- Angle-dependent phase retardation of single-scattered waves give rise to the image distortion and reduction in signal to noise ratio
- Input and output aberrations are hard to distinguish in the case of elastic scattering
References:
S. Kang et al., High-resolution adaptive optical imaging within thick scattering media using closed-loop accumulation of single scattering, Nature Communications 8, 2157 (2017)
Conjugate CLASS microscopy
We present a high-speed reflection matrix microscope using a light source with a wavelength of 1.3 μm to reduce tissue scattering and aberration. Furthermore, we develop a computational conjugate adaptive optics algorithm designed for the recorded reflection matrix to optimally compensate for the skull aberrations. These developments allow us to realize label-free longitudinal imaging of cortical myelin through an intact mouse skull. The myelination processes of the same mice were observed from 3 to 10 postnatal weeks to the depth of cortical layer 4 with a spatial resolution of 0.79 μm. Our system will expedite the investigations on the role of myelination in learning, memory, and brain disorders.

In vivo reflectance images of a mouse brain down to cortical layer 4 through an intact skull. (a) 3D-rendered through-skull image. The SHG images were used to visualize the mouse skull (colored in green). (b) Conventional OCM images at representative depths displayed with gray-dotted boxes in (a). Scale bar: 40 μm. (c) Aberration-corrected images at the same depths as those shown in (b). (d) Aberration maps of the subregions in (c). Color bar: phase in radians.
Reference:
Kwon, Y. et al. Computational conjugate adaptive optics microscopy for longitudinal through-skull imaging of cortical myelin. Nature Communications 14, 105 (2023).
Tracing multiple scattering trajectories
We developed a method to trace the multiple scattering trajectories in situ and convert them into signal waves for imaging objects of interest embedded within a scattering medium. In essence, the proposed method utilizes the measured reflection matrix to find multiple phase plates generating similar wave trajectories as the original scattering medium. In doing so, we could demonstrate in vivo through-skull imaging of a mature mouse. This study marks an important milestone in the field of deep-tissue optical imaging in that it enables the use of multiply scattered waves for microscopic image formation of an embedded object for the first time.

Tracing multiple light scattering by solving high-order inverse scattering problem. (a) Finding a set of phase plates generating similar wave propagation trajectories as the original scattering medium. (b) Finding multiple phase plates representing the mouse skull obtained from experimentally measured reflection matrix. (c) Confocal image of a mouse brain under the skull. (d) Myelinated axons in the mouse brain obtained by using multiple light scattering identified by the phase plates.
Reference:
Kang, S. et al. Tracing multiple scattering trajectories for deep optical imaging in scattering media. Nature Communications 14, 6871 (2023).